Competing with your horse is a team effort. You give 100 percent. You want your horse to give 100 percent. That’s what it takes to win. But what if the equine health care product you choose is only giving 27 percent?
Unfortunately, studies show that might be the case. Especially when it comes to products claiming to treat and/or prevent equine stomach ulcers, a condition affecting two out of three nonracing competitive horses. Horse owners need to be wary when making purchases of certain equine ulcer care product; studies and tests conducted by Scott Stanley, Ph.D., University of California—Davis and by the U.S. Food & Drug Administration (FDA) have confirmed that not all products are created equal.
And just because the product’s label contains a statement doesn’t necessarily make it so. “Horse owners should be concerned that they aren’t always getting what they are paying for,” says Megan Green, DVM, manager, large animal veterinary services, Merial. “But this has been an ongoing problem. Some manufacturers have been making claims that simply aren’t true.”
The Studies
In studies of products claiming to treat or prevent equine stomach ulcers some products were found to have inconsistent and varying amounts of the active ingredient used to treat or prevent ulcers – omeprazole. The charts below show the wide range of omeprazole found in four different “equine ulcer” products, from as little as 27 percent to as much as 126 percent.
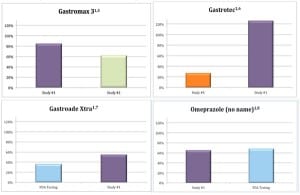
Ultimately, the FDA issued warning letters to the manufacturers of GastroMax 3 and Gastrotec, along with multiple other manufacturers.
The FDA issued these warnings because the manufacturers were marketing their unapproved animal drugs “for use in the mitigation, treatment, or prevention of disease in animals.”
Unapproved drugs have not been tested by the agency for safety or effectiveness. “The wide variation of active ingredient found in these products by Dr. Stanley and the FDA really isn’t a surprise,” says Green. “Omeprazole is highly unstable, which is why it’s so important it is manufactured under the good manufacturing practices established by the FDA.”
Besides the stability challenge inherent with omeprazole, its ability to travel through a horse’s highly acidic stomach is also a concern with unproven and unapproved drugs. “For omeprazole to work, it has to travel through the horse’s stomach, which requires a special formulation,” Green adds.
That special formulation is found in the only proven and FDA-approved products for equine stomach ulcers, which are ULCERGARD® (omeprazole) for prevention and GASTROGARD® (omeprazole) for treatment. These products are manufactured following stringent FDA guidelines, ensuring quality and efficacy.
What to Do : Work with a Veterinarian
Horse owners should always consult with their veterinarian before using equine health care products, advises Green. “Veterinarians have the most information and experience with equine health care, whether it be for treatment or preventive care. In most cases, they will also be familiar with the particular horse in question and can make educated health care recommendations.”
Check the Labels
The FDA approval process ensures a drug is safe, effective and has accurate, comprehensive labeling. In addition, following initial FDA approval, the FDA continually monitors the drug. All-FDA approved drugs for use in animals have a New Animal Drug Application (NADA) number. The six-digit NADA number and the statement “Approved by the FDA” are usually found on the drug’s label.
Unfortunately, in spite of the warning letters issued by the FDA, some illegally marketed products remain available, so horse owners should be vigilant about checking the labels to ensure they are getting products that have been proven to contain the appropriate amount of omeprazole and proven to work.
Get Educated
With all the products available to horse owners, knowing what is safe and effective is critical. One resource is the website www.equinedrugfacts.com, which helps explain the different types of products on the market and the FDA approval process.
“Horse owners have a significant investment in their horses and deserve to get what they are paying for,” says Green. “Working with a veterinarian, checking labels and becoming educated will help them make good health care decisions.”
IMPORTANT SAFETY INFORMATION:
ULCERGARD can be used in horses that weigh at least 600 pounds. Safety in pregnant mares has not been determined. Caution: Safety of GASTROGARD in pregnant or lactating mares has not been determined. For prescribing information, click here: http://www.ulcergard.com/SitecollectionDocuments/GASTROGARD_PrescribingInfo.pdf.
About Merial
Merial is a world-leading, innovation-driven animal health company, providing a comprehensive range of products to enhance the health and well-being of a wide range of animals. Merial employs 6,100 people and operates in more than 150 countries worldwide with more than €2 billion of sales in 2014. Merial is a Sanofi company. For more information, please see www.merial.com.









